
Monday: Science
What is the name for the phenomenon where a solid substance directly transitions into a gas without passing through the liquid phase?
Hint: A common example is dry ice.
Sublimation is the phenomenon where a solid substance transitions directly into a gas without passing through the liquid phase.
This process occurs under specific conditions of temperature and pressure, typically at low pressure and high temperature. Here’s how the alternative answers compare:
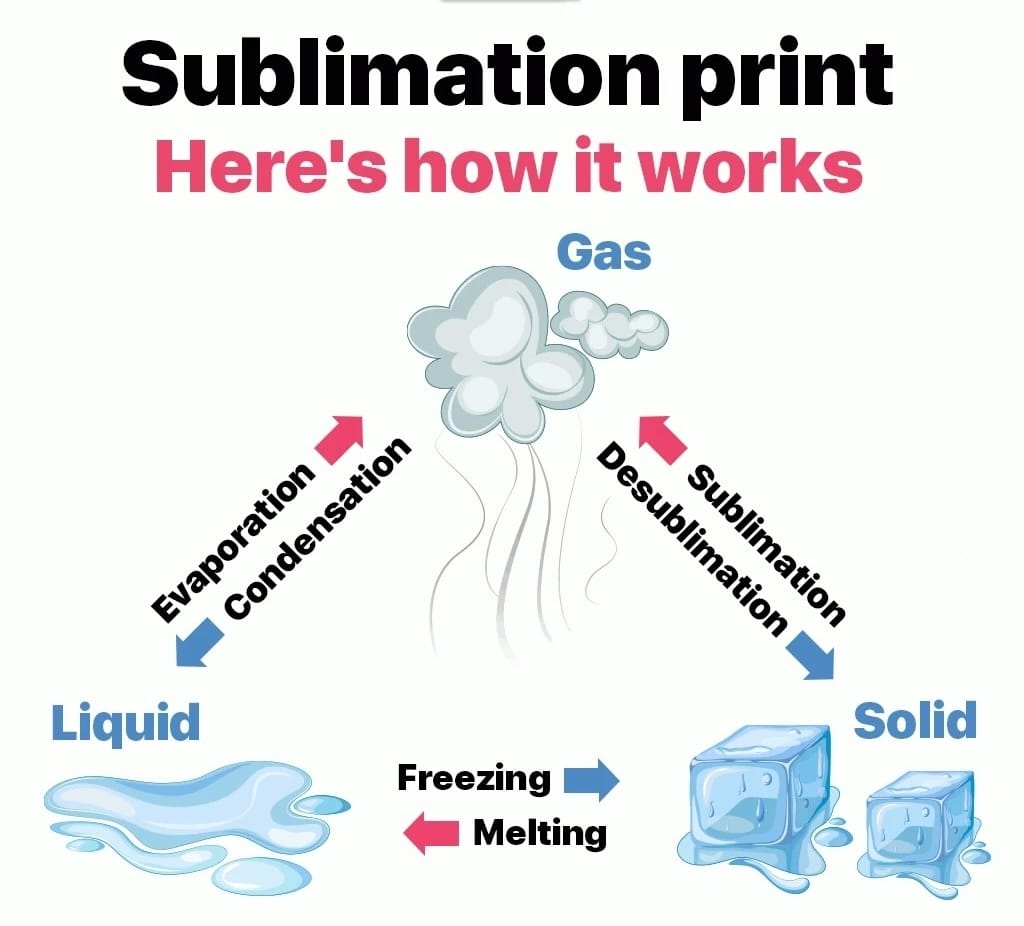
A common example of sublimation is dry ice, which is solid carbon dioxide. At room temperature, dry ice sublimates directly into carbon dioxide gas without becoming liquid. Sublimation is also observed in nature with snow and ice in cold, dry climates, where they can vaporize directly into water vapor. This unique phase change plays a crucial role in various industrial processes, including freeze-drying and the purification of substances.
